Clathrates
THE POISON IS THE CURE:
Clathrates, Methane Trigger, Alt.fuels & Telluric Currents
The global warming/environmental risk in clatrates is in NOT mining them. Researchers in Alaska have successfully drilled gas hydrates -- frozen methane deposits that could someday replace petroleum as a key energy source.
http://news.mongabay.com/2007/0222-hydrates.html
Methane hydrate can only form naturally in sediments at reasonably high pressures and low temperatures. Deposits can escape spontaneously from the ocean bed and their plumes of effervescent bubbles, perhaps up to 20-30 feet in diameter, have been blamed for otherwise unexplained disappearances of ships and rising methane might even stall aircraft by disturbing oceanic or atmospheric density.
Every now and then, chunks of these erupting clouds of methane hydrates break free and rise rapidly to the surface and into the atmosphere. Submarine gas releases from the sea floor could, in theory, sink ships with giant bubbles which change the bouyancy of some vessels. Whether or not the ship sinks depends of its relative position to the bubbles. The methane plume is basically a low-density foam that creates turbulence.
But this is not the only global threat they pose. Methane has a much greater capacity to trap heat in the atmosphere than carbon. When it converts from a solid or ice state to a gaseous state a methane blow-out occurs as a natural phenomenon.
METHANE TRIGGER: Methane is a powerful greenhouse gas which, despite its atmospheric lifetime of around 12 years, none the less has a global warming potential of 62 over 20 years and 21 over 100 years (IPCC, 1996; Berner and Berner, 1996; vanLoon and Duffy, 2000). The sudden release of large amounts of natural gas from methane clathrate deposits is suggested as a cause of past and possibly future climate changes. Events possibly linked in this way are the Permian-Triassic extinction event, the Paleocene-Eocene Thermal Maximum.
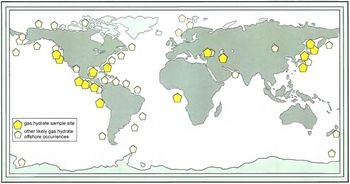
Methane clathrate, also called methane hydrate or methane ice, is a solid form of water that contains a large amount of methane within its crystal structure (clathrate hydrate). Originally thought to occur only in the outer regions of the solar system where temperatures are low and water ice is common, extremely large deposits of methane clathrate have been found under sediments on the ocean floors of Earth. Hydrates only form in a narrow range of depths such as those of continental shelves.
http://en.wikipedia.org/wiki/Methane_hydrate
THE THREAT: 165w, 55n Clathrate Trigger
http://www.aeic.alaska.edu/Seis/recenteqs_sub/quakes/2007030_evid153578/evid153578.html
600 Million Years Ago Methane Release:
What's New with My Subject?
Anomalies caused by ancient event
By KEVIN HOWE
Herald Staff Writer
Global warming is nothing new.
It ended the last great ice age 10,000 to 12,000 years ago, and the effects of that warming are still being felt today, according to ocean geologists with the Monterey Bay Aquarium Research Institute.
Geologists Charles Paull and William Ussler spent the late summer and early fall of 2003 aboard a Canadian icebreaker plying the Beaufort Sea off Canada's north coast to examine a geological anomaly, "pingo-like" objects on the sea floor similar to rounded hill formations called pingos that are found on the surface in the arctic ice.
Their findings, published last month in the scientific journal Geophysical Research Letters, indicate the objects are being pushed up by methane gas released after long-buried gas hydrates -- icelike deposits of methane that occur under extremely cold conditions -- began decomposing when the melting polar ice cap caused sea levels to rise and inundate the continental shelves.
Over the ensuing millennia, Paull said, the warmer water -- close to freezing but about 20 degrees Celsius warmer than the gas hydrates -- sent a heat pulse downward and began thawing the gas.
"There's a lot of interest in the scientific community about gas hydrates," he said. "These are compounds that most people have never seen, and yet we argue they're common on earth and may be a very important compound on earth.
"Humans and gas hydrates are incompatible. We die under the conditions they form in. They decompose quickly. It comes as an eye-opener; it may be a very important phase on earth we just haven't experienced."
Chemists in the late 1800s theorized that gas hydrates may exist, Paull said, and they were observed in pipelines in the 1930s, but weren't discovered in nature until the 1960s.
Gas hydrates are distinct from the more familiar liquid hydrocarbons found in oil fields, Ussler said. "It's a relatively new material, found on the ocean floor, primarily methane."
The two scientists said there are several practical areas of interest involving the gas hydrocarbons:
• Methane is a greenhouse gas that could contribute to global warming, and gas hydrates are found worldwide, not just in the arctic. While release of methane began with the end of the ice age, scientists wonder if current global warming might accelerate the process. That would have the effect of increasing global warming, Ussler said, "a process of positive feedback; it builds on itself and goes faster.
"The downside is that there's really not much we can do about it. The train has left the station in terms of momentum and we can't stop the propagation of the thermal pulse down into seafloor, but knowing what the outcome might be makes things a little less pleasant." Climate scientists, Ussler said, predict the permanent loss of arctic ice over the next century.
• Gas hydrates are found worldwide. In addition to those found under permafrost in the polar regions, Ussler said, they have been found on the west and east coast of United States, in the Gulf of Mexico and in the Mediterranean.
"Almost everywhere we've looked and had the tools to observe and detect them, we've found them. The more we explore, the more we realize how extensive they are."
• Decomposing gas hydrate deposits could make the sediments in the continental shelf areas where offshore oil drilling rigs operate unstable, Paull said, and threaten these installations.
• As a potential source of fuel, methane is a clean-burning gas that produces carbon dioxide on combustion, a gas absorbed by plants which convert it to oxygen. At present, Ussler said, the cost of extracting it profitably is probably too high for the energy industry.
Paull noted, however, that the United States, Canada, Japan, Korea and India have programs exploring whether they can become a long-term energy source.
A. San-Miguel, P. Kéghélian, X. Blase, P. Mélinon, A. Perez, J. P. Itié, A. Polian, E. Reny, C. Cros, and M. Pouchard
Phys. Rev. Lett. 83, 5290
(issue of 20 December 1999)
http://whyfiles.org/119nat_gas/2.html
<!--[if !supportEmptyParas]--> <!--[endif]-->
http://www.du.edu/~jcalvert/econ/hydrates.htm
The evanescent existence of dodecahedra in liquid water would seem to facilitate the formation of gas hydrates.
<!--[if !supportEmptyParas]--> <!--[endif]-->
http://www.du.edu/~jcalvert/econ/hydrates.htm
mechanism for clathrate chain reaction is same as mjr's moab
but more powerful; uses chlorine hydrate, not oxygen, takes place in water layer between 2 plates--instant super pressure steam.
imagin a huge, huge bellows -shaped volcano laying on its side with nozzle under unimak island pointing south. unimak behaves as part of upper plate and is mainly a marker.
more than enough to trigger super quake- and reliably, too.
you wind up with a pool of atomic hydrogen combining with atomic chlorine
the most powerful chemical exxplosive known.
the aptly named HAL came up with the specific info that removing aPREVIOUSLY UNKNOWN massive hydrate deposit at 165w 55n would delay thechain reaction methanogenesis that is the climate trigger.<!--[if !supportEmptyParas]--> <!--[endif]-->interrogating HAL , he reluctantly spit out a 1981 doc from a BIOLOGICALsurvey which was wrongly thought to map a huge colony of methaneproducing organisms but in fact mapped a relic deposit of clathratewhich is about to slide off the alaska shelf in an area that generatedthe 1946 mega-tsunami.....at 165w 55n near unimak island in thealeutians.<!--[if !supportEmptyParas]--> http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=16471893&dopt=Abstract
Gas hydrates of argon and methane synthesized at high pressures: composition, thermal expansion, and self-preservation.
Nikolaev Institute of Inorganic Chemistry SB RAS, Prospekt Akad. Lavrentieva 3, Novosibirsk 630090, Russian Federation.
For the first time, the compositions of argon and methane high-pressure gas hydrates have been directly determined. The studied samples of the gas hydrates were prepared under high-pressure conditions and quenched at 77 K. The composition of the argon hydrate (structure H, stable at 460-770 MPa) was found to be Ar.(3.27 +/- 0.17)H(2)O. This result shows a good agreement with the refinement of the argon hydrate structure using neutron powder diffraction data and helps to rationalize the evolution of hydrate structures in the Ar-H(2)O system at high pressures. The quenched argon hydrate was found to dissociate in two steps. The first step (170-190 K) corresponds to a partial dissociation of the hydrate and the self-preservation of a residual part of the hydrate with an ice cover. Presumably, significant amounts of ice Ic form at this stage. The second step (210-230 K) corresponds to the dissociation of the residual part of the hydrate. The composition of the methane hydrate (cubic structure I, stable up to 620 MPa) was found to be CH(4).5.76H(2)O. Temperature dependence of the unit cell parameters for both hydrates has been also studied. Calculated from these results, the thermal expansivities for the structure H argon hydrate are alpha(a) = 76.6 K(-1) and alpha(c) = 77.4 K(-1) (in the 100-250 K temperature range) and for the cubic structure I methane hydrate are alpha(a) = 32.2 K(-1), alpha(a) = 53.0 K(-1), and alpha(a) = 73.5 K(-1) at 100, 150, and 200 K, respectively.
PMID: 16471893 [PubMed - in process]
| ||||||||||||||||||||||
No, not The Blob, but something perhaps far more sinister: methane, a potent greenhouse gas 30 times better than carbon dioxide at trapping atmospheric heat.
Research released Thursday finds that underground methane appears to be seeping through the Arctic Ocean floor and into the Earth's atmosphere, thanks to a weakening of the protective layer of permafrost at the bottom of the ocean. Once released into the atmosphere, methane could wreak havoc with the world's climate.
Although scientists have known about the methane under the Arctic— and its potential for leakage — since the 1990s, the study is the first to document it to this degree.
"The release to the atmosphere of only 1% of the methane assumed to be stored in shallow hydrate deposits" could increase the level of atmospheric methane worldwide by three or four times, says the study's lead author, Natalia Shakhova, a researcher at the University of Alaska-Fairbanks. That could trigger abrupt climate warming, the authors report. But the specific climate consequences are hard to predict, they say.
The researchers studied the East Siberian Arctic Shelf, a section of the Arctic Ocean just north of Siberia.
Historically, methane concentrations in the world's atmosphere have ranged between 0.3 and 0.4 parts per million in cool periods to 0.6 to 0.7 in warm periods. Current methane concentrations in the Arctic average about 1.85 parts per million, the scientists said, the highest in 400,000 years.
"The amount of methane currently coming out of the East Siberian Arctic Shelf is comparable to the amount coming out of the entire world's oceans," Shakhova says. "Subsea permafrost is losing its ability to be an impermeable cap."
Permafrost, which occurs throughout the Arctic, is a layer of soil or rock at which the temperature has been continuously below 32 degrees for at least several years, according to the National Snow and Ice Data Center.
Methane is released when organic material in the thawing permafrost decomposes, which gradually releases methane. It also can be released directly as stored methane already in the permafrost is released as it thaws.
Why is the permafrost failing? According to the study, the process occurs naturally over thousands of years but is being accelerated by man-made climate warming. "Sustained release of methane to the atmosphere from thawing Arctic permafrost is a likely positive feedback to climate warming," the authors write in the study, which appears in the new edition of the journal Science.
The East Siberian Arctic Shelf, in addition to holding large stores of frozen methane, is an additional concern because it is so shallow. In deep water, methane gas oxidizes into carbon dioxide before it reaches the surface. In the shallow East Siberian Arctic Shelf, methane simply doesn't have enough time to oxidize, which means more of it escapes into the atmosphere.
This is a new topic, Shakhova concedes, and the findings are only a starting point for further study.
"We're at the very beginning of studying this topic," Shakhova says. "This has never been incorporated into climate models."
Contributing: Associated Press